Research
The Hyatt Research Group is dedicated to advancing synthetic chemistry for scientific progress and human benefit. We have a long-standing history of investigating the properties of hypervalent iodine, and applying our mechanisms to pharmaceutical synthesis. In recent years, we have also expanded our research to include the synthesis of dental antibiotics and high temperature superconductivity.
Hypervalent Iodine Chemistry
What is hypervalency?
When chemists call something “hypervalent” it means that the normal rules of bonding do not apply. Typically, nonmetals follow the octet rule; they prefer to form structures with eight shared electrons around each element. Hypervalency is when compounds form with over eight electrons. In our group, we use iodine(III) which has 10 electrons in most structures. In this higher oxidation state, the hypervalency can be used to exploit unusual and often unexplored metal-like properties of this commonly encountered nonmetal.
Why Iodine?
Most people are familiar with the element iodine due to its popularity as an antiseptic sold as iodine tinctures. These alcohol containing solutions cause iodine to have a brown color through the generation of something called a charge transfer complex. If iodine is not in an alcohol solution, if forms a different charge transfer complex with air that makes it take on a purple color. However, when in a vacuum, iodine looks like a silvery metal (see images below).

Iodine is a diverse element and exploring the metal-like properties of compounds containing iodine in its hypervalent state is the main focus of the Hyatt Research Group. Though often used in literature, especially with drug design and synthesis, contributions to research in hypervalent iodine (HVI) are only undertaken by a small community of chemists. Given iodine(III)’s metallic properties, we hypothesize that HVI can facilitate the formation of carbon-carbon bonds while avoiding β-hydride elimination. This is feasible through transmetallation, as iodine can undergo transmetallation with organometalloids and organometallics. Addressing this issue is significant because iodine cannot perform β-hydride elimination.
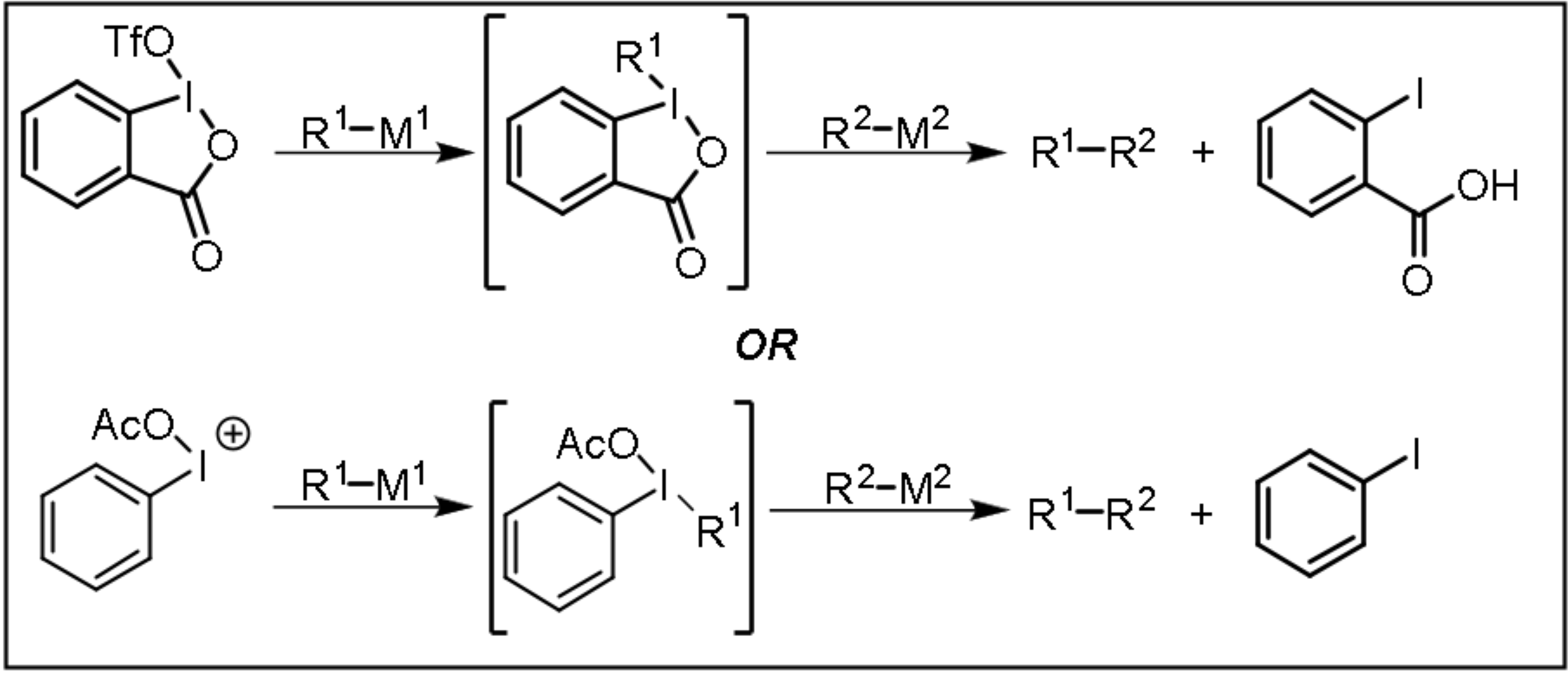 Carbon-Carbon Bond Formation Theory
Carbon-Carbon Bond Formation Theory
The Hypervalent Iodine Transmetallation (HIT) mechanism can be used to explain the double-displacement reaction that takes place with organometalloids, organometallics, and any hybridization of carbon. HIGES (read more below) explains how iodine’s metal-like character guides regioselective reactions.
- Hypervalent Iodine-Guided Electrophilic Substitution
- Formation of Spirolactams for Trichomoniasis Vaginalis
Antibiotic Dental Composites
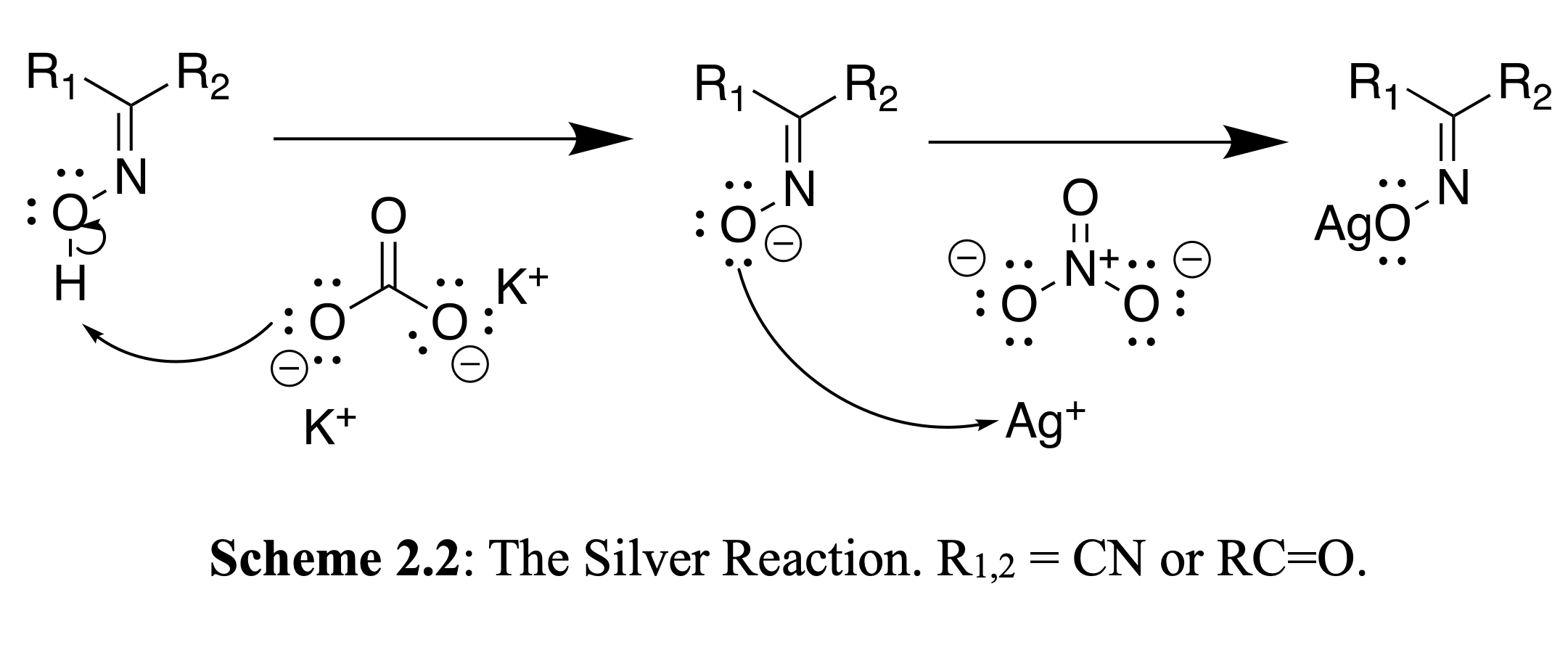
Silver is used to purify water and treat medical related issues such as urinary tract infections. Our undergraduate researchers have applied this to the synthesis of silver oximate antimicrobial compoounds for dental science in the treatment of cavities. Synthesized compounds have been tested on biological specimens, yielding positive results.
High Temperature Superconductivity
Using state-of-the-art machine learning algorithms and techniques, we are committed to using artificial intelligence to further the field of superconductivity. We have assembled equipment to refine the synthesis of existing cuprate superconductors, such as the famous YBCO compound, and synthesize new compounds we theorize by our computational workflow.